K-bio names draw limelight on progress in precision therapies in lung cancer forum
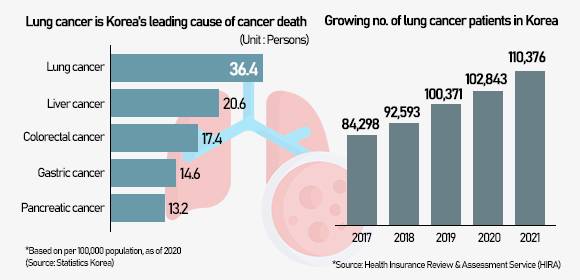
EGFR or epidermal growth factor receptor is a protein on cells that helps them grow. It is identified as being highly expressed in cancer cells including lung cancer, a leading cause of cancer death in Korea. A mutation in the EGFR gene can make it grow too much, which can cause cancer and resistance to existing EGFR tyrosine-kinase inhibitors. About 40 percent of lung cancer patients in Asia, including Korea, develop lung cancer due to mutations in the EGFR gene.
Some Korean biopharmas grabbed attention for their impressive clinical trial results with advanced EGFR-targeted cancer drugs that have overcome treatment resistance.
Audience was pack at Yuhan Corp.¡¯s presentation on the results of first-line monotherapy with Leclaza (lazertinib mesylate monohydrate). All seats were full in a session on overcoming resistance to existing EGFR targeted therapies, which was held in the largest conference room there, where two Korean biotech firms, Bridge Biotherapeutics and J INTS BIO, made a presentation.
![[Photo by IASLC 2022 World Conference on Lung Cancer,]](https://img3.daumcdn.net/thumb/R658x0.q70/?fname=https://t1.daumcdn.net/news/202208/12/mk/20220812142102865ohcj.jpg)
Bridge Biotherapeutics unveiled interim results of a Phase 1 clinical trial of BBT-176, its fourth-generation EGFR-targeted cancer drug, at the conference. The fourth-generation version is being developed for patients whose cancer recurs due to resistance to third-generation therapies such as Tagrisso or Leclaza. No company in the world has yet succeeded in commercializing a fourth-generation EGFR-targeted cancer drug.
[¨Ï Maeil Business Newspaper & mk.co.kr, All rights reserved]
Copyright © 매일경제 & mk.co.kr. 무단 전재, 재배포 및 AI학습 이용 금지
- LG Chem to invest nearly $400 million to upgrade and expand ABS plastic facilities - Pulse by Maeil Business News Korea
- Foreign capital dominates Korean M&A with ample funds and stronger USD vs KRW - Pulse by Maeil Business News Korea
- NCSoft, Krafton Q2 OP off nearly 50% on qtr, Netmarble turns red - Pulse by Maeil Business News Korea
- Korean fintech platform Toss operator nears getting new ammunition for banking growth - Pulse by Maeil Business News Korea
- Korea’s sea flag carrier HMM should be gradually privatized: Oceans Min - Pulse by Maeil Business News Korea
- 강경준, 상간남 피소…사랑꾼 이미지 타격 [MK픽] - 스타투데이
- AI가 실시간으로 가격도 바꾼다…아마존·우버 성공 뒤엔 ‘다이내믹 프라이싱’- 매경ECONOMY
- 서예지, 12월 29일 데뷔 11년 만에 첫 단독 팬미팅 개최 [공식] - MK스포츠
- 이찬원, 이태원 참사에 "노래 못해요" 했다가 봉변 당했다 - 스타투데이
- 양희은·양희경 자매, 오늘(4일) 모친상 - 스타투데이