29 out of 5.3 million people exhibit allergic reaction after being vaccinated in US, CDC says
이 글자크기로 변경됩니다.
(예시) 가장 빠른 뉴스가 있고 다양한 정보, 쌍방향 소통이 숨쉬는 다음뉴스를 만나보세요. 다음뉴스는 국내외 주요이슈와 실시간 속보, 문화생활 및 다양한 분야의 뉴스를 입체적으로 전달하고 있습니다.
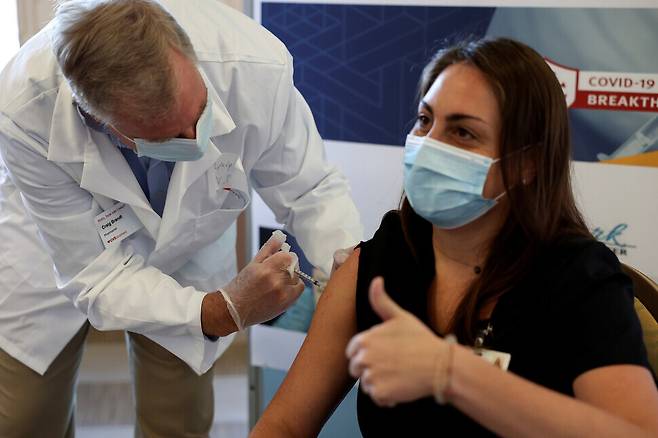
At least 29 people have exhibited a severe allergic reaction after receiving a COVID-19 vaccination in the US, where some 5.3 million people have been vaccinated to date. The US Centers for Disease Control and Prevention (CDC) urged people to undergo vaccination, stressing that serious allergic reactions have been rare and that no deaths have occurred.
On Jan. 6, the Wall Street Journal reported on the CDC’s announcement that symptoms of anaphylaxis were observed in at least 29 out of the 5.3 million or so people inoculated with a COVID-19 vaccine as of Jan. 5. Anaphylaxis is a severe allergic reaction in which contact with even small amounts of a particular substance such as a vaccine or food results in symptoms such as breathing difficulty and skin rashes. The vaccines administered in the US to date were produced by Pfizer and BioNTech and Moderna, and no fatalities have been reported thus far.
As a ratio, the number of people exhibiting symptoms of anaphylaxis amounted to 5.5 in one million, or four times higher than the 1.3 for flu vaccinations. But Nancy Messonnier, director of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), stressed that in terms of actual numbers, cases of anaphylaxis were still exceedingly rare.
“[The COVID-19 vaccine is] a very safe vaccine, and we're in the setting of 2,000 COVID deaths per day,” she said, adding that “the risk of poor outcomes, especially in senior citizens, makes it imperative that people go ahead and get vaccinated as soon as it’s available to them.”
But public health officials in various countries are stressing the need for caution in vaccinating people with a history of serious allergic reactions. June Raine, chief executive of Britain’s Medicines and Healthcare Products Regulatory Agency (MHRA), said last year that “any person with a history of anaphylaxis to a vaccine, medicine or food should not receive the Pfizer BioNTech vaccine.”
Messonnier said that anyone who exhibits an immediate allergic reaction to the first dose should not receive a second one. She also stressed that anyone with a history of allergic reactions should be observed for around 30 minutes after receiving the vaccine, and that institutions should have a system in place for responding to allergic reactions.
By Cho Ki-weon, staff reporter
Please direct comments or questions to [english@hani.co.kr]
Copyright © 한겨레. 무단전재 및 재배포 금지.
- 개의 가축화, ‘단백질 중독’ 피하려 남는 살코기 늑대 주다 시작됐다?
- 그날 밤 ‘강남 4구’만 교통지옥으로 변한 까닭은
- 트럼프, 의사당 난입 사태로 처벌받나?…검찰의 수사 대상으로
- 추미애 “동부구치소 집단감염…방역지침 따라 적절 조처 다했다”
- 대뜸 “아이 잘있나요?”…정인이 사건 후 입양가정 ‘낙인’에 위축
- 정은경 “11월까지 코로나 집단 면역 목표”
- 눈길에선 벤츠보다 강하다? 야쿠르트 카트의 비밀
- 최씨는 왜 폭설에도 ‘신차 운송’에 나서야만 했을까
- [한국갤럽] 문 대통령 국정수행 부정 평가 55%…긍정은 38%
- 법원 “반인도적 행위…‘위안부’ 피해자에게 1억원씩 배상”